Clinical Trial Management
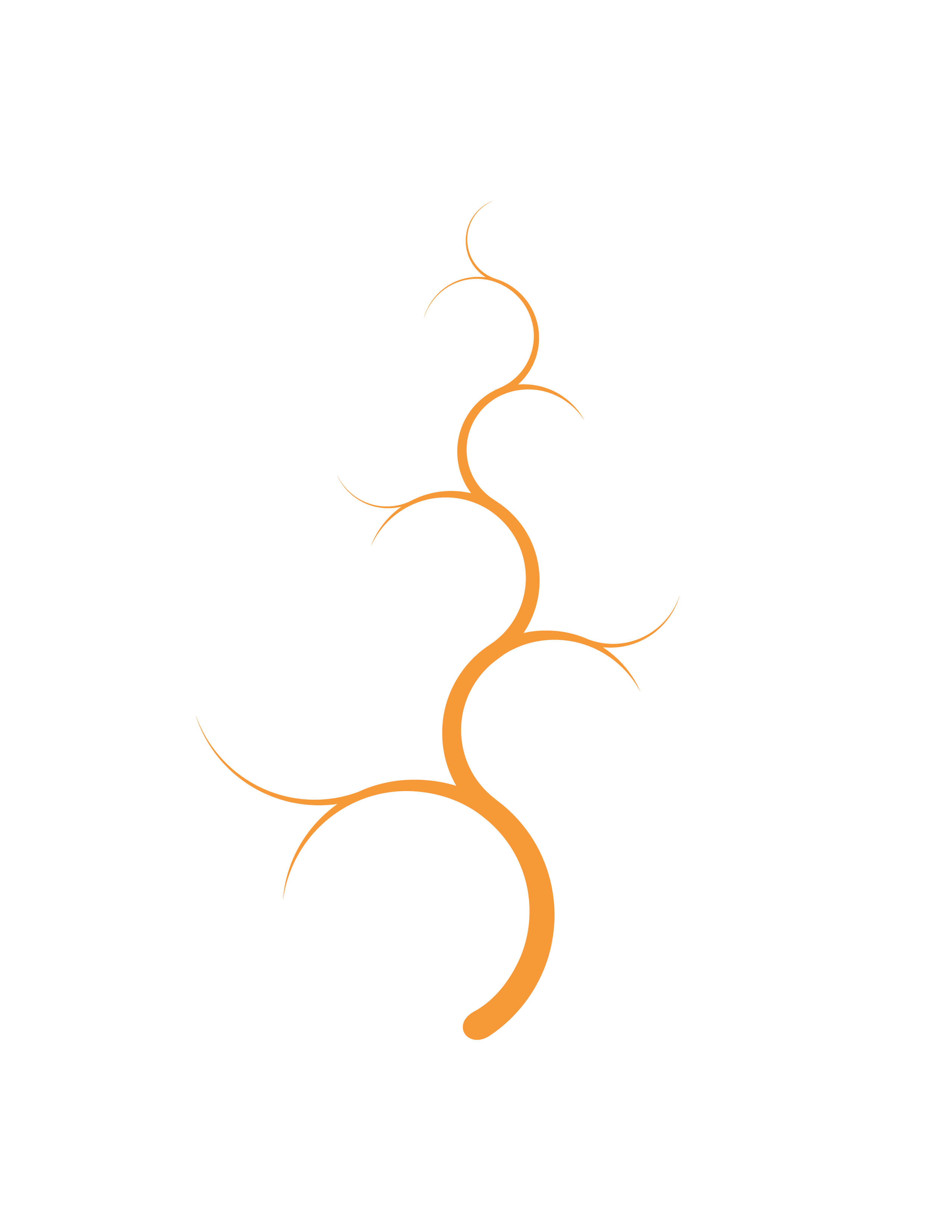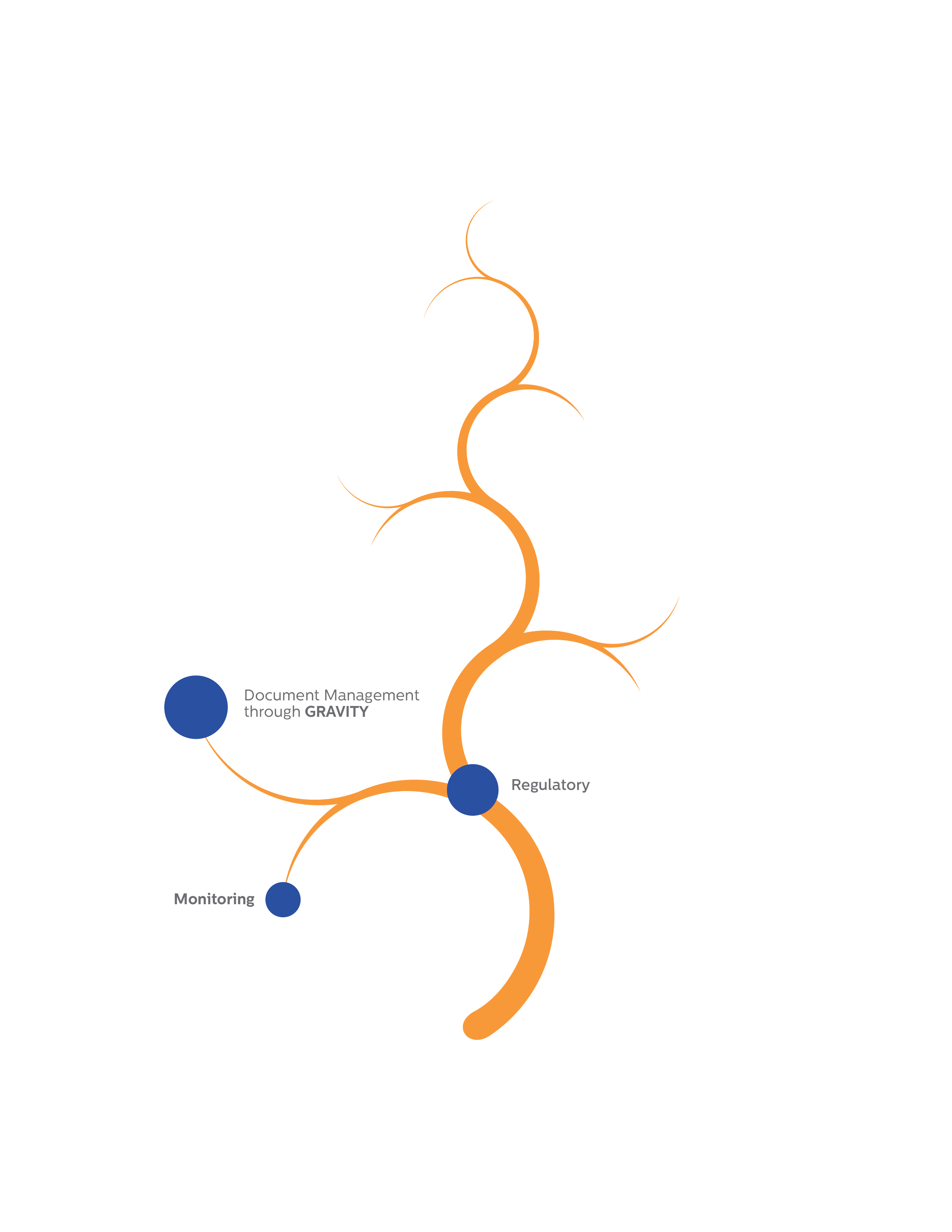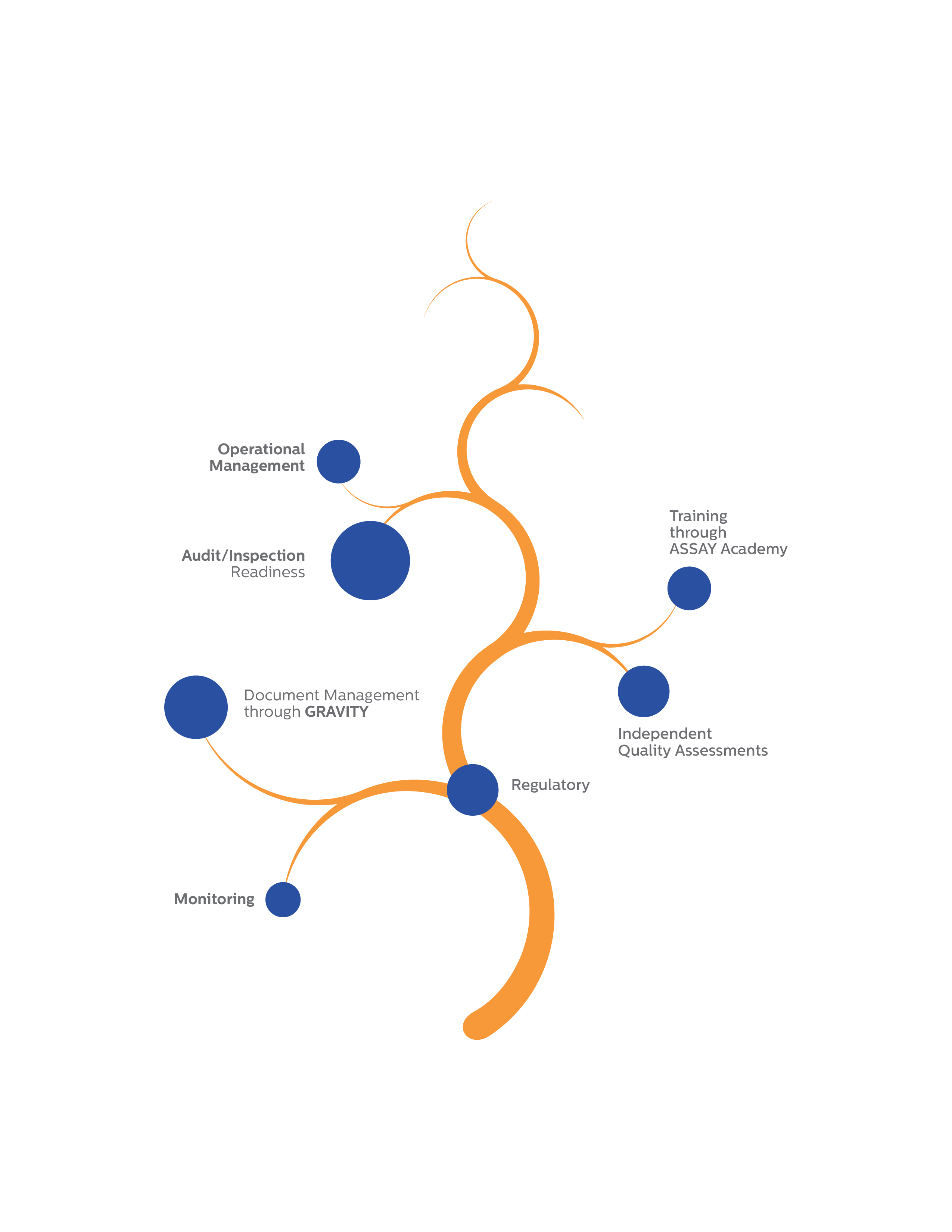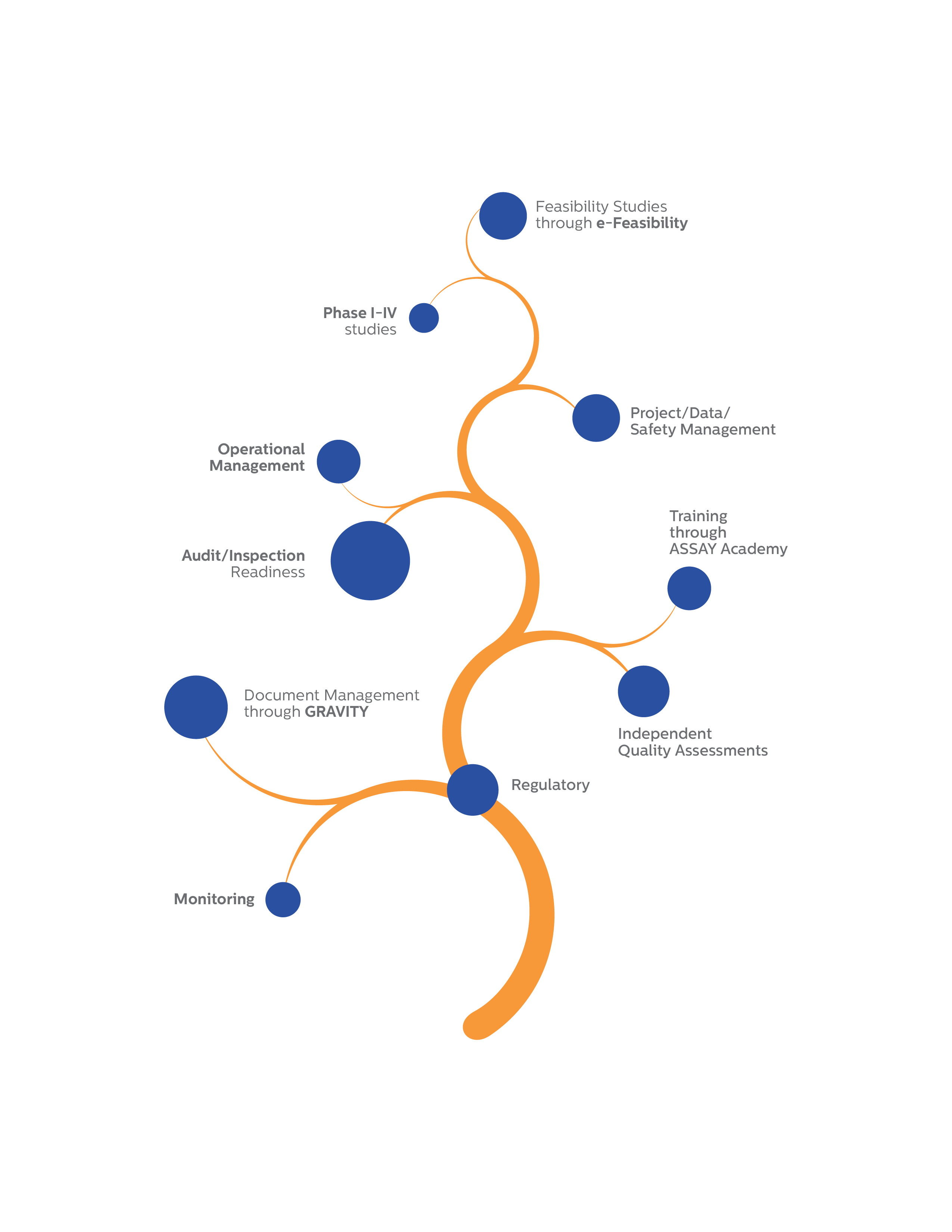
Feasibility Studies through
e-Feasibility
Phase I-IV studies
Project/Data Safety Management
Operational Management Team
Audit/Inspection Readiness
Training through ASSAY Academy
Independent Quality Assessments
Document Management through
GRAVITY
Monitoring
Regulatory